Ceramics and glasses dry perfectly well in the dishwasher, but plastic items seem to remain steadfastly wet, forcing the dishwasher unloader to either dry the plastic items using a tea towel or, more hygienically, leaving the plastic items to air-dry on a clean and sanitised draining board. Have you ever wondered why the plastic items refuse to dry when the other items will readily be exsiccated?

First of all, what do we mean by plastic? In this context I think we mean low density polyethylene (LDPE) or polypropylene (PP). The word plastic really refers to the material property where the material exhibits permanent deformation when deforming forces are removed. Plastic is the opposite of elastic, which means a material exhibits only temporary deformation and returns to its original shape when deforming forces are removed. That isn’t terribly relevant to this post though, so let us move on.
Dinner plates, bowls and cups can be made from ceramic materials, such as porcelain, stoneware, but also a cheaper material called melamine. I had never heard of melamine before, but it looks like a hard ‘plastic’ (colloquial use of the word) that is used to make ‘unbreakable’ dinnerware. I’m just going to focus on four materials in this post. Here is some relevant data, according to engineeringtoolbox.com, designerdata.nl and matweb.com:
Stoneware has a specific heat capacity of 800 J/(kg°C) and a density of 2350 kg/m³
Porcelain has a specific heat capacity of 1070 J/(kg°C) and a density of 2300 kg/m³
LDPE has a specific heat capacity of 2600 J/(kg°C) and a density of 925 kg/m³
PP has a specific heat capacity of 1700 J/(kg°C) and a density of 910 kg/m³
Comparing these data could be tricky, but if we assume that the items in the dishwasher are all plates that are the same physical dimensions, we could compare the materials by calculating a volumetric specific heat capacity (also just called the volumetric heat capacity); the amount of energy that must be transferred per unit volume to cause a 1°C temperature change. As we have assumed the physical dimensions of the items are the same, the volumes will be the same, so the numerical comparisons would be more useful. To calculate this volumetric specific heat capacity, we must multiply the specific heat capacities by the densities for each material.
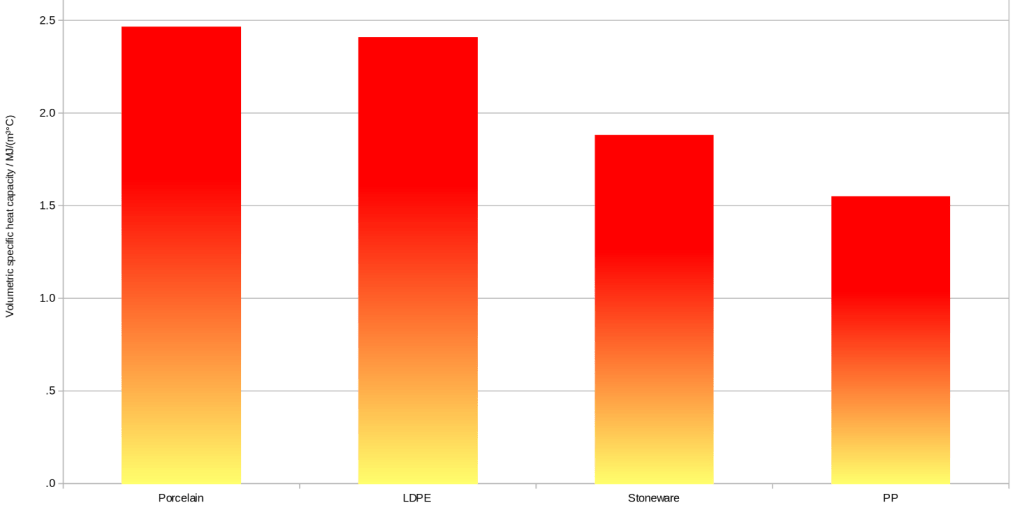
Stoneware has a volumetric specific heat capacity of 1.88 MJ/(m³°C)
Porcelain has a volumetric specific heat capacity of 2.461 MJ/(m³°C)
LDPE has a volumetric specific heat capacity of 2.405 MJ/(m³°C)
PP has a volumetric specific heat capacity of 1.547 MJ/(m³°C)
Immediately after the dishwasher stops, there is water on the surface of the items inside it. Water that is at a higher temperature evaporates more quickly. The graph below shows the evaporation rate (in grams of water that evaporates per unit surface area of the item the water is on per second) versus the temperature of the water (in °C). The water will be the same temperature as the item is it on because energy is transferred by conduction between the water and the item, although the time it takes for the water and item to reach thermal equilibrium depends on the thermal conductivity of the material from which the item is made. As the items and the water have been in the dishwasher for some time during the wash cycle, I think it is a fair assumption that they are at the same temperature when the dishwasher program stops.

As the water on the item evaporates, it will reduce the temperature of the item. This is because the more energetic water molecules will leave the surface of the water droplets, reducing the mean kinetic energy of the water molecules that remain. The temperature of the water is proportional to the mean kinetic energy of the water molecules. Energy transfers by conduction from the item to the water, thus the item’s temperature reduces until the item and the water droplets are again at thermal equilibrium.
For PP, the item will need to transfer less energy to reduce the temperature by 1°C, so the temperature of the PP will reduce more rapidly than the temperature of the porcelain. As the temperature of the porcelain remains higher for longer, the water will evaporate from the porcelain more quickly, so it will dry sooner.
That explains why the porcelain items can be taken out of the dishwasher dry, by PP items are still wet. What about LDPE versus stoneware? Here, the volumetric specific heat capacity of the stoneware is actually lower than that for LDPE, so what’s going on here?
Our initial simplification (that the items were of the same physical dimensions) is the issue here. LDPE items will tend to be thinner than stoneware items. Stoneware items have to be thick because of how brittle stoneware is. In order to have stoneware that is strong enough to survive daily use, it must be significantly thicker than the LDPE polymer items. While I am writing this, I do not have my micrometer with me, but if I were to estimate, I would say the stoneware would be at least five times thicker.
This means that even though LDPE has a higher volumetric specific heat capacity (28% higher), the items have less volume (20% of the volume), so the heat capacity of the LDPE items is lower than the stoneware items (LDPE items’ heat capacity is 72% of the stoneware items’ heat capacity). Heat capacity is just the amount of energy that must be transferred per unit temperature change. The LDPE items’ temperatures will drop more quickly than the stoneware because the heat capacity of the LDPE items is lower than the heat capacity of the stoneware items.

Interestingly, if the LDPE and stoneware items did have the same volume, the surface-area-to-volume-ratio of the LDPE items is higher than for the stoneware items because the LDPE items are thinner. The evaporation rate on the above graph was ‘per unit surface area‘, so the larger surface area of the LDPE items means a higher evaporation rate. I want to test this, but that means getting two items, one made from LDPE and one made from stoneware, that are the same volume but different thicknesses, stick them in a dishwasher, and see if the LDPE item dried first.
There are a lot of different factors that affect the drying rate of items in the dishwasher; their geometry, specific heat capacity and the thermal conductivity are three of such factors. To have a fair test, we would need to conduct an experiment where only one of these variables was volitionally changed, but actually we were interested in why my plastic items do not dry when my ceramic items do, and my plastic items are far thinner and occupy far less volume than my ceramic items, so I guess I will have to be satisfied with that answer.
By the way, exsiccated means dried. Yup, I used a thesaurus.
Reminder: Weekly Live Tuition Sessions!
SATURDAY 7th March 2026
| GCSE Physics | 9:30am | Gas laws |
| A-Level Physics | 10:30am | Wave basics |
| GCSE Astronomy | 11:30am | Formation of planetary systems (2) |
If you wish to enrol on the tuition sessions and haven’t yet, then click enrol below